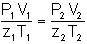COMPRESSIBILITY
is a volume ratio that indicates the deviation (as a multiplier) of the actual volume from that as determined by the perfect or ideal gas laws. When compressibility is applied, the equation is the real gas law. Compressibility is designated by the term “z”, and is a function of pressure, temperature, and gas composition.